Medical Body Area Network’s
Medical Body Area Network’s
Medical Body Area Network (MBAN) technology will provide a flexible platform for the wireless networking of multiple body transmitters used for the purpose of measuring and recording physiological parameters and other patient information or for performing diagnostic or therapeutic functions, primarily in health care facilities. This platform will enhance patient safety, care and comfort by reducing the need to physically connect sensors to essential monitoring equipment by cables and wires. As the numbers and types of medical radio devices continue to expand, these technologies offer tremendous power to improve the state of health care in the United States.
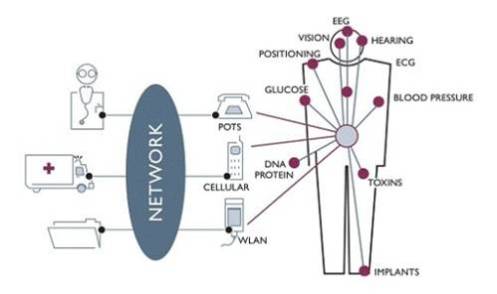
An MBAN is a little like a cellular wireless system in miniature, worn on a patient’s body. Sensors around the body monitor various functions, depending on the patient’s needs, and communicate their data to a central hub, worn by the patient or located close by. The hub aggregates data from the various sensors, and transmits those data using the health care facility’s network (possibly over Wi-Fi or Ethernet) to a central control point, from where the data are made available to the professional staff for interpretation and appropriate response.
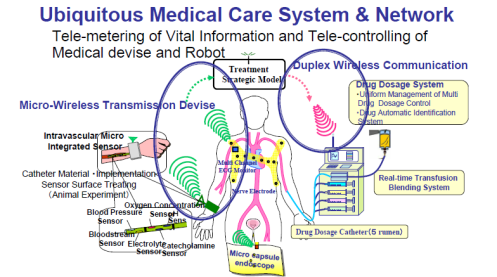
Medical applications of BAN cover continuous waveform sampling of biomedical signals, monitoring of vital signal information, and low rate remote control of medical devices. They can be broadly classified into two categories depending on their operating environments. One is the wearable BAN, which is mainly operated on the surface or in the vicinity of the body, such as medical monitoring. Another is the implantable BAN, which is operated inside the human body, e.g. capsule endoscope and pacemaker.
Wired Technologies
Wired technologies inevitably result in reduced patient mobility and increased difficulty and delay in transporting patients. Caregivers, in turn, can spend inordinate amounts of time managing and arranging monitor cables, as well as gathering patient data.
The introduction of MBAN represents an improvement over traditional medical monitoring devices (both wired and wireless) in several ways, and will reduce the cost, risk and complexity associated with health care. For example, a health care facility could monitor more patients, particularly in patient care areas where Wireless Medical Telemetry Service (WMTS) is not currently installed; an MBAN could be used outside the health care facility, e.g., within patients’ homes; and an MBAN could be used for both monitoring and therapeutic applications.
Wireless Technologies
Wireless Medical Telemetry Service (WMTS) in health care facilities has overcome some of the obstacles presented by wired sensor networks. Nonetheless, WMTS is an in-building network that is often used primarily for monitoring critical care patients in only certain patient care areas. The MBAN concept would allow medical professionals to place multiple inexpensive wireless sensors at different locations on or around a patient’s body and to aggregate data from the sensors for backhaul to a monitoring station using a variety of communications media.
Currently, there are multiple frequency bands available for different types of wireless medical device applications. The MedRadio service provides an umbrella framework to regulate the operation of both implanted and body-worn wireless medical devices used for diagnostic and therapeutic purposes in humans. MedRadio uses spectrum in the 401-406 MHz, 413-419 MHz, 426-432 MHz, 438-444 MHz, and 451-457 MHz bands, all on a secondary basis.
The Wireless Medical Telemetry Service (WMTS) allows for the transmission of patient-related telemetric medical information to a central monitoring location within a hospital or other medical facility. WMTS operates in the 608-614 MHz, 1395-1400 MHz, and 1427-1432 MHz bands on a primary basis. In addition, medical radio device manufacturers have for many years been allowed to market products which operate on a variety of frequencies on an unlicensed basis.
Dedicated Medical Body Area Networks (MBANs) Wireless Spectrum
The FCC recently took regulatory action (MBAN Joint Proposal) to dedicate spectrum for wireless monitoring sensors, or Medical Body Area Networks (MBANs), making the U.S. the first country in the world to dedicate spectrum specifically for wireless health devices. The FCC concluded that the only cost resulting from these new regulations is the risk of increased interference, and have minimized that risk by adopting rules that permit an MBAN device to operate only over relatively short distances and as part of a low power networked system.
Initially Aeronautical Mobile Telemetry (AMT) licensees and the MBAN proponents – parties strongly disagreed as to whether MBAN and AMT operations could successfully coexist in the same frequency band. The approach taken will permit providing frequencies where an MBAN can co-exist with existing spectrum users and engage in robust frequency re-use, which will result in greater spectral efficiency.
FCC MBAN Joint Proposal
This FCC Joint Proposal is a comprehensive plan that draws from both the existing MedRadio and WMTS rules to specify MBAN operational requirements for body-worn sensors and hubs. It includes a detailed set of requirements for MBAN management within a health care facility. It also proposes that MBAN use in the 2360-2390 MHz band be limited mostly to indoor use and subject to specific coordination procedures and processes to protect AMT users in that band, whereas MBAN use in the 2390-2400 MHz band could occur at any location and without coordination.
The Joint Proposal describes an MBAN as consisting of a master transmitter (hereinafter referred to as a “hub”), which is included in a device close to the patient, and one or more client transmitters (hereinafter referred to as body-worn sensors or sensors), which are worn on the body and only transmit while maintaining communication with the hub that controls its transmissions. The hub would convey data messages to the body-worn sensors to specify, for example, the transmit frequency that should be used.
The hub and sensor devices would transmit in the 2360-2400 MHz band. The hub would aggregate patient data from the body-worn sensors under its control and, using the health care facility’s local area network (LAN) (which could be, for example, Ethernet, WMTS or Wi-Fi links), transmit that information to locations where health care professionals monitor patient data. The hub also would be connected via the facility’s LAN to a central control point that would be used to manage all MBAN operations within the health care facility.
To protect AMT operations from harmful interference, the Joint Proposal would have the Commission designate an MBAN frequency coordinator who would coordinate MBAN operations in the 2360-2390 MHz band with the AMT frequency coordinator. The control point would serve as the interface between the MBAN coordinator and the MBAN master transmitters to control MBAN operation in the 2360-2390 MHz band. The control point would receive an electronic key, which is a data message that specifies and enables use of specific frequencies by the MBAN devices. The control point, in turn, would generate a beacon or control message to convey a data message to the hub via the facility’s LAN that specifies the authorized frequencies and other operational conditions for that MBAN.
MBAN – Licensed by Rule
To help encourage the development of MBAN devices and applications, the FCC decided not to require users to apply for and receive individual licenses from the FCC. Instead, all MBANs will be “licensed by rule,” which means that users will be deemed licensed as long as they abide by all technical and operational limitations.
However, MBAN operations will be permitted only on a secondary basis — users must not cause harmful interference to and must accept interference from the primary licensees in the band.
MBAN Power and Frequency Summary
The permitted MBAN operations depend on which portion of the band will be used:
- MBANs using the 30 MHz in the 2360-2390 MHz band. MBAN operations in this band are restricted to indoor uses in health care facilities. Users of this portion of the band will be required to register with an MBAN coordinator (discussed below, will be selected at a later date) and coordinate with primary licensees, if necessary.
- MBANs using the 10 MHz in the 2390-2400 MHz band. In this band, MBAN operations can be used in any location, such as in a health care facility, in a patient’s home, or outdoors while the patient is in transit (e.g. ambulances). Users of this portion of the band will not be subject to registration and coordination requirements.
- Power limits are relatively generous: 1 milliwatt at 2360-2390 MHz, with a higher limit of 20 milliwatts at 2390-2400 MHz, in part to give patients greater mobility within their homes.
FDA Role in MBAN Functions
In the past, the Food and Drug Administration (“FDA”) has expressed concern about the potential for interference when health care providers rely on wireless medical devices. In a 2007 draft guidance document, Radio-Frequency Wireless Technology in Medical Devices, FDA commented that a quality system for devices that incorporate wireless technology should address potential concerns such as wireless quality of service, wireless coexistence, data integrity and security, and applicable EMC and telecommunications standards and regulations. As a result, there is likely to be increased collaboration between the FCC and the FDA as wireless medical devices enter the market. In particular, the FCC suggested that the FDA could play an important role in specifying whether MBANs may be used to perform functions that are life-critical or time-sensitive.
Wireless Coexistence
Although there is some overlap between electromagnetic compatibility (EMC) and wireless coexistence, differences exist. Wireless coexistence is the ability of one wireless system to perform a task in an environment where other systems that may or may not be using the same set of rules can also perform their tasks.EMC is the ability of a device to function properly in its intended electromagnetic environment without introducing excessive electromagnetic energy that could interfere with other devices. Manufacturers of electrically powered medical devices routinely test their equipment to applicable national and international consensus safety standards. EMC test results are often used to support safety claims to regulatory agencies such as FDA. Less well-known are the issues and concerns associated with wirelessly enabled medical devices, although this is changing thanks to FDA’s guidance document on wireless medical devices.
At any given time, a typical home or hospital uses a number of wireless systems (e.g., IEEE 802.11a/b/g/n, or WiFi; Bluetooth; ZigBee; cordless phones) operating on the same industrial, scientific, and medical (ISM) band. Given the increasing use of wireless, RF wireless medical devices and other wireless systems operating nearby can interfere with each other. If a collision between their respective transmissions occurs, data packets transmitted by medical devices could be delayed or blocked, potentially interfering with timely transmissions of critical data. Techniques such as retransmission and forward error correction might no longer be sufficient to overcome interference and spectrum congestion. Hence, methods to design and test wirelessly enabled medical devices for risks associated with coexistence of wireless technologies are essential for innovative, safe, and effective RF wireless medical devices.
Summary
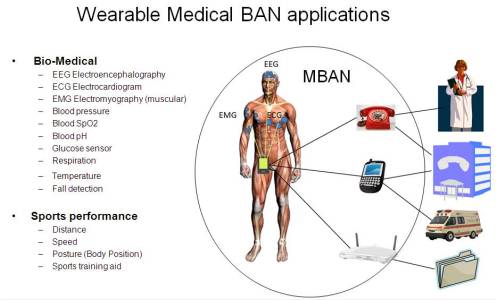
There is a need for ultra low power devices and operation on, in or around the human body to serve a variety of applications including medical and personal entertainment. Examples of the applications are: Electroencephalogram (EEG), Electrocardiogram (ECG), Electromyography (EMG), vital signals monitoring (temperature (wearable thermometer), respiratory, wearable heart rate monitor, wearable pulse oximeter, wearable blood pressure monitor, oxygen, pH value, wearable glucose sensor, implanted glucose sensor, cardiac arrhythmia), wireless capsule endoscope (gastrointestinal), wireless capsule for drug delivery, deep brain stimulator, cortical stimulator (visual neuro-stimulator, audio neuro stimulator, Parkinson’s disease, etc…), remote control of medical devices such as pacemaker, actuators, insulin pump, hearing aid (wearable and implanted), retina implants, disability assistance, such as muscle tension sensing and stimulation, wearable weighing scale, fall detection, aiding sport training. This will include body-centric solutions for future wearable computers.
In a similar vein, the same technology can provide effective solutions for personal entertainment as well. The existence of medical body area networks will provide opportunities to expand these product features, better healthcare and well being for the users. It will therefore result in economic opportunity for technology component suppliers and equipment manufacturers.
Medical applications are critical applications and may be life critical. The requirements for medical Body Area Networks (BANs) include: robust links for bounded data loss and bounded latency, capacity for high density of patients and sensors, coexistence with other radios, battery life for days to months of continuous operation, and small form factors for body devices.
These requirements can be satisfied through the utilization of a number of techniques including error control techniques and adaptive repeat requests, low duty cycle and power management, and the development of more efficient, diverse antennas.
Existing standards have been designed for commercial applications with little or no consideration for life saving emergency scenarios. In particular, there is a need to ensure reliable communications by network devices such as sensors that are involved in emergency situations, while ensuring low power consumption.